
SAN FRANCISCO — A combination of chemotherapy and antibody therapy was effective and well tolerated in patients with previously untreated chronic lymphocytic leukemia (CLL), according to a study at the M.D. Anderson Cancer Center in Houston.
Fludarabine (Fludara, Berlex) and cyclophosphamide (Cytoxan, Bristol-Myers Squibb) made up the chemotherapy part of the regimen. Rituximab (Rituxan, Genentech and IDEC) was the antibody used.
---Michael J.
Keating, MB, BS
“The combination is the most active regimen explored by our group in
previously untreated CLL,” said Michael J. Keating, MB, BS, who presented
the information here at the 42nd Annual Meeting of the American Society of
Hematology.
The study enrolled 68 patients with previously untreated CLL at progressive or advanced-stage disease. The median patient age was 58 years, which was younger than the typical CLL patient, said Keating, a professor of medicine at the M.D. Anderson Cancer Center in Houston and a member of the Hem/Onc Today Editorial Advisory board.
All patients received 25 mg/m2 of fludarabine and 250 mg/m2 of cyclophosphamide daily for three days for six courses every four weeks. They received 375 mg/m2 of rituximab on day 1 of the first course. On day 1 of the remaining courses, patients received 500 mg/m2 of rituximab. Treatment was administered on an out-patient basis.
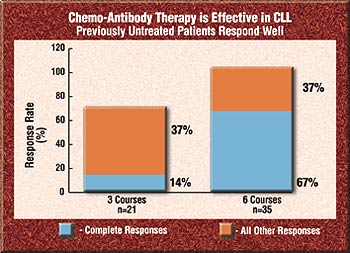
At the time of the presentation, 35 patients had completed the six courses. Twenty-one patients who had completed only three courses were included in the response evaluation.
Rituximab is an anti-CD20 antibody. Other studies have shown it has dose-related efficacy in CLL and seemed to sensitize lymphoid cells to chemotherapy.
“The addition of rituximab to the fludarabine-cyclophosphamide combination is effective because the fludarabine enhances the ability of the rituximab to kill CLL cells. The fludarabine and cyclophosphamide also appear to be synergistic,” Keating told Hem/Onc Today.
Response rate
Keating and his colleagues noted a high response rate after three courses. The overall response at that point was 81%. After six courses, the overall response rate increased to 94%.
After three courses, 14% of the 21 patients had achieved a complete response. After six courses, 57% of 35 patients had a complete response.
“The FCR results are likely to be associated with longer remission durations and a greater number of patients having five- to 10-year remissions,” Keating said.
Keating said he would recommend a six-course therapy for this group of patients.
Toxicity
 The toxicity of FCR was similar to that expected with
fludarabine-cyclophosphamide, Keating said.
The toxicity of FCR was similar to that expected with
fludarabine-cyclophosphamide, Keating said.
Although 61% of patients experienced grade-1 to grade-2 toxicities, he said it did not significantly impact their quality of life, as these toxicities occurred only during the days of therapy. Grade-3 to grade-4 toxicities occurred in 14% of patients.
Reactions to rituximab were not common after the first course of therapy.
The fludarabine-cyclophosphamide combination produced nausea in 21% of courses, with associated vomiting in 7% of total courses. Major infections included pneumonia and septicemia and occurred in 3% of courses. One patient died of pneumonia after the second course of therapy.
Minor infections included fevers of unknown cause, herpes and soft tissue infections. These occurred in about 10% of all courses. There were no opportunistic infections.
The most common hematologic toxicity was neutropenia, which led to fludarabine-cyclophosphamide reductions in 21% of patients.
Keating plans to continue studying FCR and perhaps introduce other agents into the combination in future trials.
For more information:
- Keating MJ, O’Brien S, Lerner S, et al. Combination chemo-antibody therapy with fludarabine (F), cyclophosphamide (C) and rituximab (R) achieves a high CR rate in previously untreated chronic lymphocytic leukemia (CLL). Abstract #2214. Presented at the 42nd Annual Meeting of the American Society of Hematology. Dec. 1-5, 2000. San Francisco.